SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic

responding covid-19 pandemic
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic
What Wikipedia / Google Says
About ‘Coronavirus Covid-19 Pandemic‘ ? :
Portraying All Stories
ON/ABOUT/FROM
‘Coronavirus Covid-19 Pandemic’
Add Story/ Your Say Until the Cause is Concluded
[*Select Category/Tag:
Coronavirus Covid-19 Pandemic
at Your Next Publish Screen.]

Johns Hopkins Coronavirus Resource Center
CBS News: What is a coronavirus testing desert?
CBS News correspondent Mark Strassmann spoke with Jennifer Nuzzo, a senior scholar at the Johns Hopkins Center for Health Security, about areas where people don’t have access to coronavirus tests, known as testing deserts.
as per our monitoring this Story originally appeared * : ) here → *
Johns Hopkins Coronavirus Resource Center
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic

Doctors behind viral COVID-19 misinformation video met with Vice President Pence
The Week
Flynn suggests Trump deploy the military in ‘swing states’ to ‘rerun’ the election, soft-pedals martial law
Former National Security Adviser Michal Flynn tiptoed once again toward urging President Trump to declare martial law to overturn President-elect Joe Biden’s victory, citing discredited voter fraud claims. Trump “could immediately, on his order, seize every single one of these voting machines,” Flynn told Newsmax host Greg Kelly Thursday night. “He could order the, within the swing states, if he wanted to, he could take military capabilities, and he could place those in states and basically rerun an election in each of those states.”Using the U.S. military to force states to redo an election is “not unprecedented,” Flynn added. “These people are out there talking about martial law like it’s something that we’ve never done. Martial law has been instituted 64 times, Greg. So I’m not calling for that. We have a constitutional process,” and “that has to be followed.”> Here’s Michael Flynn on Newsmax saying that Trump could order “military capabilities” to swing states and “rerun an election in each of those states.”> > “People out there talk about martial law like it’s something that we’ve never done. Martial law has been instituted 64 times.” pic.twitter.com/KNmiAGGiPF> > — Aaron Rupar (@atrupar) December 18, 2020The federal government hasn’t implemented martial law since after Japan bombed Pearl Harbor in 1941, and then only in the territory of Hawaii. “Flynn’s insane rant” appears to rely on “the numerous invocations of martial law” before and during the Civil War, University of Texas law professor Steve Vladek said. Since then, Supreme Court precedents and several laws — notably the Posse Comitatus Act — have severely constrained the president’s ability to declare martial law.”Nothing to see here,” Slate’s Will Saletan tweeted. “Just a retired general and former undeclared foreign agent for an authoritarian regime, freshly pardoned by the president for lying to the FBI about his contacts with Russia, asserting precedents for martial law to overturn the president’s electoral defeat.”Former President Barack Obama fired Flynn as head of the Defense Intelligence Agency, then “tried to warn Trump about him,” New York Times reporter Maggie Haberbman noted. “Trump hired him, fired him, complained about him and his son during transition, and then has obviously changed course.” The Bulwark’s Tim Miller added: “Michael Flynn lying to the FBI was the biggest break the nation caught during the Trump years. The thought of the insane mad man in the room when Trump was making decisions is frightening.”More stories from theweek.com 5 insanely funny cartoons about Trump’s election-fraud failure Trump has reportedly been convinced he actually won, tells advisers he may not vacate the White House Pfizer says ‘millions’ of vaccine doses are waiting to be shipped — but the government hasn’t told them where to go
as per our monitoring this Story originally appeared * : ) here → *
Doctors behind viral COVID-19 misinformation video met with Vice President Pence
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic
Russia wants to distribute its questionable coronavirus vaccine to other countries: report
 © Mikhail Svetlov/Getty Images Russian President Vladimir Putin Mikhail Svetlov/Getty Images
© Mikhail Svetlov/Getty Images Russian President Vladimir Putin Mikhail Svetlov/Getty Images
- Russian President Vladimir Putin wants to distribute its questionable Sputnik V coronavirus vaccine to other countries, Reuters reported Saturday.
- Russia first approved Sputnik V in August, but experts and health officials were skeptical the vaccine would work since it did not go through necessary phase 3 trials.
- The Kremlin also kept important information relevant to the vaccine’s success out of the public’s eye. This included its methodology.
- Visit Business Insider’s homepage for more stories.
Russian President Vladimir Putin said Saturday that the country is hoping to distribute its controversial Sputnik V coronavirus vaccine to other countries, according to Reuters.
Russia announced a successful coronavirus vaccine in August, but Sputnik V was approved under questionable circumstances. It was released before it went through phase 3 trials. In the United States, phase 3 is a requirement before a drug or vaccine can be vetted and approved by the Food and Drug Administration.
As Business Insider’s Susie Neilson previously reported, the vaccine’s early-trial results had not undergone peer review. Russia had also not revealed its methodology, further enshrouding Sputnik V in secrecy.
The rushed timeline led health officials to speculate whether the Kremlin coerced vaccine makers into putting out Sputnik V quickly to gain a leg up in the global race for a cure to the coronavirus.
Speaking at the annual G20 Summit, Putin said Saturday that Russia is in the process of creating a second and third vaccine in response to the coronavirus, Reuters reported Saturday.
Video: ‘Bursting with antibodies’: U.K. prime minister in quarantine again (NBC News)
What to watch next
UP NEXT
Putin also said restated his goal to mass-produce the vaccine for other countries. For months, the Russian president has been pushing other countries to take his vaccine seriously.
Russia in August said it would start the mass production process in September, despite the uncertainty that plagued Sputnik V.
Earlier this month, the Kremlin announced that preliminary data showed the vaccine had a 92% effectiveness rate at preventing the coronavirus. But the data was based on just 20 confirmed COVID-19 cases, according to a press release.
Putin told news reporters in August that he had offered to help the United States develop a coronavirus vaccine, but the White House declined.
Putin, however, maintains the assertion that the vaccine has “passed all the needed checks,” even adding at one point that he had his own daughter take it.
There are two coronavirus vaccines that have proven to have a high success rate at fighting the coronavirus. Pharmaceutical company Pfizer and biotech group Moderna announced earlier this month that they’ve developed vaccines with at least a 94.5% success rate at preventing the coronavirus in clinical trials.
Read the original article on Business Insider
Microsoft may earn an Affiliate Commission if you purchase something through recommended links in this article.
as per our monitoring this Story originally appeared * : ) here → *
Russia wants to distribute its questionable coronavirus vaccine to other countries: report
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic
Indiana coronavirus updates for Thursday, Dec. 10, 2020
INDIANAPOLIS — Thursday’s latest updates on the coronavirus pandemic:
IPS does not plan to return to in-person learning on January 4
Indianapolis Public Schools released the following statement regarding the Marion County Health Department’s announcement that in-person learning can resume on January 4.
“IPS does not anticipate shifting back to in-person learning on January 4 at this time. We believe it will be critical to assess the conditions and data after the Winter Break in order to make a determination if it is safe to return to in-person learning prior to the already scheduled January 19 date.
The safety of our students and staff continues to be our top priority. We will continue to leverage our return to in-person learning framework approved by the Board of School Commissioners in October.”
Marion County leaders giving update
Indianapolis Mayor Joe Hogsett and MCPHD Director Dr. Virginia Caine are giving an update on COVID-19 in Marion County.
Hogsett opened by saying the city needs help from the federal government to keep families financial afloat during the pandemic, noting that there has been no follow-up to the CARES Act passed in March. The funds distributed from the CARES Act will stop at the end of the year. He encouraged people to contact the leaders of Congress and demand action.
Dr. Caine recommended students and teachers stay away from people outside their household for 10 days before returning to in-person schooling next year. Caine said the executive order on closing schools was initially set to go through Jan. 18, but now schools may return to in-person learning on Jan. 4.
Caine said her office felt comfortable continuing to have bars open at 25 percent capacity after looking at the statistics and science, while restricting education to virtual only.
Ellen DeGeneres tests positive
Talk show host Ellen DeGeneres announced Thursday that she has tested positive for COVID-19.
“Fortunately, I’m feeling fine right now. Anyone who has been in close contact with me has been notified, and I am following all proper [Centers for Disease Control and Prevention] guidelines,” DeGeneres said in a statement posted on Twitter.
“I’ll see you all again after the holidays. Please stay healthy and safe,” she said in her statement.
ISDH daily update
The Indiana State Department of Health is reporting 6,604 more positive cases and 96 additional deaths from COVID-19. That brings Indiana’s totals to 404,935 cases and 6,302 confirmed deaths. Another 301 deaths are considered “probable,” meaning COVID-19 is believed to have contributed to a patient’s death, but there was no positive COVID test result on file.
The positivity rate remains high: 13.9 percent for all tests between Nov. 27 and Dec. 3, and 26.7 percent for unique individuals in the same time span.
More than 43 percent of the state’s ICU beds are in use by COVID patients. There are just over 20 percent of ICU beds available. On Wednesday, there were 3,221 people being treated for coronavirus in Indiana hospitals.
Moderna begins testing COVID-19 vaccine in US adolescents
Moderna announced Thursday that it has started testing its coronavirus vaccine candidate in adolescents.
The company, which made one of two vaccines expected to be approved soon for adult use in the U.S., said it plans to enroll 3,000 U.S. kids as young as 12 in the trial.
So far, there have only been a handful of attempts around the world to start exploring if any of the experimental coronavirus shots being pushed for adults also can protect children. Some U.S. pediatricians are worried they may not know if any of the shots work for young children in time for the next school year.
In a statement, Moderna Chief Executive Officer Stéphane Bancel said they hope to have data in the spring that can support giving the COVID-19 vaccine to children before the start of the 2021 school year.
Each child in the Moderna trial will either receive the COVID-19 candidate or a placebo at both vaccinations.
Indiana house speaker tests positive
Indiana House Speaker Todd Huston has tested positive for COVID-19. He got the result Wednesday and is experiencing mild symptoms.
“Unfortunately, I have tested positive for COVID-19,” Huston said in a statement. “I will continue quarantining at home and taking all necessary precautions. I look forward to returning to work when it’s safe to do so.”
Huston has not been in contact with any colleagues or staff members. He has not been to the Indiana Statehouse in the last week.
US jobless claims jump to 853,000
The number of people applying for unemployment aid jumped last week to 853,000, the most since September, evidence that companies are cutting more jobs as new virus cases spiral higher.
The Labor Department said Thursday that the number of applications increased from 716,000 the previous week. Before the coronavirus paralyzed the economy in March, weekly jobless claims typically numbered only about 225,000.
The latest figures coincide with a surging viral outbreak that appears to be weakening the job market and the economy and threatening to derail any recovery. Consumers thus far haven’t spent as much this holiday shopping season as they have in previous years, according to credit and debit card data. And in November, employers added jobs at the slowest pace since April. Restaurants, bars and retailers all cut jobs last month.
The total number of people who are receiving state-provided unemployment aid rose for the first time in three months to 5.8 million, the government said, from 5.5 million. That suggests that some companies have sharply pulled back on hiring.
All told, more than 19 million people are still dependent on some type of unemployment benefit. And unless Congress acts soon, nearly half of them will lose that aid in just over two weeks. That’s when two jobless aid programs that the federal government created in the spring are set to expire.
Indiana reinstating surgery limits amid COVID-19 surge
Indiana Governor Eric Holcomb is reimposing restrictions on hospitals from performing elective surgeries to free up hospital capacity with the state’s steep recent increases in serious COVID-19 illnesses.
Holcomb announced Wednesday that hospitals were being directed to postpone all non-urgent in-patient surgeries beginning Dec. 16 through Jan. 3.
Holcomb said Indiana is “on fire” with coronavirus spread.
The state halted elective medical procedures in April to help preserve hospital equipment and protective gear. Holcomb said he was extending the statewide mask order and toughening restrictions on crowd sizes that he reinstated last month.
Marion Co. COVID-19 update scheduled for 2 p.m.
Indianapolis Mayor Joe Hogsett and Marion County Public Health Department Director Dr. Virginia Caine will provide an update regarding Marion County’s current COVID-19 data on Thursday, Dec. 10 at 2 p.m.
WTHR will stream the news conference live here and on Facebook.
Pfizer COVID-19 vaccine faces last hurdle before US decision
Pfizer’s COVID-19 vaccine faces one final hurdle before an expected decision to greenlight the shot for use in millions of Americans. Food and Drug Administration advisers meet Thursday to scrutinize the company’s data for any red flags or oversights.
The public review comes as U.K. regulators investigate two apparent cases of allergic reaction to the vaccine. Safety will be top of mind for the panel of medical experts, who will vote on whether to endorse the vaccine.
They will also address unknowns about the vaccine’s effectiveness in certain groups. A final FDA decision and the first shots could follow within days.
Latest US, world numbers
There have been more than 15.3 million confirmed cases of COVID-19 in the U.S. as of 3:30 a.m. ET Thursday, according to Johns Hopkins University. There have been more than 289,400 deaths and 5.88 million people recovered.
Worldwide, there have been more than 68.9 million confirmed cases with more than 1.57 million deaths and 44.4 million recoveries.
The real number of people infected by the virus around the world is believed to be much higher — perhaps 10 times higher in the U.S., according to the Centers for Disease Control and Prevention — given testing limitations and the many mild cases that have gone unreported or unrecognized.
For most people, the coronavirus causes mild or moderate symptoms. For some, especially older adults and people with existing health problems, it can cause more severe illness like pneumonia, or death.
Carmel tests wastewater to predict COVID-19 spikes
The City of Carmel continues to test its wastewater to determine the prevalence of Covid-19 infections within the community. These studies can show an increase of the virus in the community up to 10 days ahead of a possible spike in positive cases. Wastewater samples are collected at the Carmel Wastewater Treatment Plant at 96th St. and the White River, then analyzed for the virus.
Mayor Jim Brainard said the city is taking this extra step to help local hospitals respond to a growing number of Covid-19 patients. Test data will allow hospitals to anticipate staffing needs and allocation of pharmaceuticals, along with scheduling elective procedures.
To test for COVID-19, copies of viral RNA (DNA) are pulled from the wastewater and quantified so that researchers may trend the numbers and determine if there are spikes or decreases in the wastewater concentrations.
as per our monitoring this Story originally appeared * : ) here → *
Indiana coronavirus updates for Thursday, Dec. 10, 2020
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic

How soon can Michiganders get a COVID-19 vaccine? Answering your biggest questions
CLOSE![]()
Dr. Anthony Fauci said distribution of vaccines for the coronavirus could begin by December or early January. USA TODAY
In the midst of another deadly coronavirus surge in a pandemic that has already taken more than 267,000 American lives, including 9,134 in Michigan, there’s hope on the horizon in promising news about potential COVID-19 vaccines.
Massachusetts-based biotech company Moderna applied Monday for emergency use authorization from the U.S. Food and Drug Administration for its coronavirus vaccine candidate on the heels of more encouraging data from late-stage clinical trials that suggest it is 94% effective and prevents severe illness in people with COVID-19.
Moderna is the second company to request federal approval for a potential coroanvirus vaccine. Pfizer — with its German-based partner BioNTech — asked regulators for emergency use authorization Nov. 20 for its coronavirus vaccine candidate.
Clinical trials suggest Pfizer’s vaccine is 95% effective against COVID-19 beginning 28 days after the first dose.
How soon can I get a COVID-19 vaccine?
An FDA advisory committee is expected to meet Dec. 17 to review the potential Moderna vaccine’s safety and efficacy, the company announced Monday. If it’s approved, the first doses of the vaccine could be given as early as Dec. 21, the New York Times reported.
The same committee is scheduled to discuss the Pfizer COVID-19 vaccine candidate Dec. 10. Pfizer said in a news release that its vaccine could be ready for distribution “within hours” of getting approval.
“We could be seeing both of these vaccines out and getting into people’s arms before Christmas,” Alex Azar, secretary of the U.S. Department of Health and Human Services, said Monday on “CBS This Morning.”
But that doesn’t mean most people will be able to call their doctors to get a vaccination before the New Year.
There will be limited supply — especially initially — of any vaccine that gets emergency use authorization. It could be months before there’s enough available for the general public to get immunized.
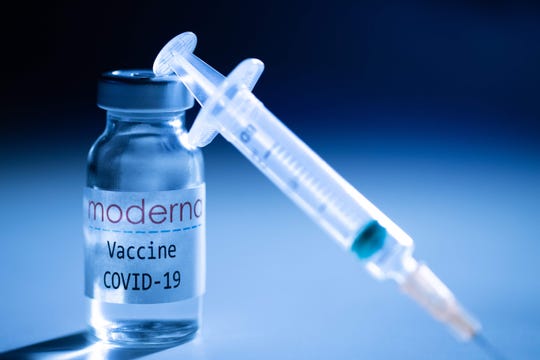
Moderna’s experimental COVID-19 vaccine on Nov. 16, 2020, in Paris. (Photo: Joel Saget/AFP via Getty Images)
How effective are these COVID-19 vaccines?
Though neither company has published outcomes of its Phase 3 trials, early data from that research suggests the COVID-19 vaccine candidates are very effective.
In its Phase 3 trial of 30,000 people, Moderna’s vaccine candidate was shown to be 94.1% effective and prevents severe illness in people with COVID-19.
Of the 196 coronavirus cases evaluated among the people who took part in the trial, 185 were in the placebo group. Additionally, of the 30 people enrolled in the Moderna vaccine trial who had severe cases of COVID-19, all 30 were in the placebo group.
Clinical trials of Pfizer’s vaccine candidate suggest it is 95% effective against COVID-19 beginning 28 days after the first dose. Of 170 confirmed cases of COVID-19 evaluated in its Phase 3 trials of the Pfizer vaccine, the company reported 162 were among people in the group that got placebo. Eight cases were identified in the group that received the vaccine.
Are they safe?
Though safety reviews continue, so far no serious concerns have been identified for either Pfizer’s or Moderna’s COVID-19 vaccine candidates.
The most common side effects were injection site pain/redness, fever, fatigue, muscle and joint aches, and headache. Patients in the Moderna trials said the reactions were more pronounced after the second dose of the vaccine.
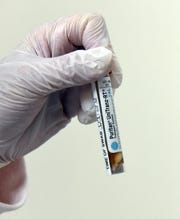
A vial of Pfizer’s mRNA COVID-19 Vaccine is prepared for first dosing at University of Maryland School of Medicine. (Photo: University of Maryland School of Medicine)
There was one COVID-19-related death in the Moderna vaccine study, but the person who died was in the group who received placebo rather than vaccine.
How many doses of the vaccine will each company produce?
Pfizer says it will produce up to 50 million doses of its vaccine globally in 2020 and up to 1.3 billion doses by the end of 2021.
Moderna also is scaling up its global manufacturing with an aim of delivering 500 million to 1 billion doses per year, beginning in 2021.
While those seem like huge numbers, it’s not enough to vaccinate the world’s population of roughly 7.8 billion people, which means doses will be distributed using priorities established by federal and state governments.
Additionally, both vaccines require two doses per person.
Who will get vaccinated first?
The U.S. Centers for Disease Control and Prevention is working with state public health officials to plan for distribution of COVID-19 vaccines.
The CDC’s Advisory Committee on Immunization Practices is planning an emergency session Tuesday to vote on a recommendation that health care providers should be vaccinated first, followed by residents of long-term care facilities, according to a STAT News report.
The Michigan Department of Health and Human Services drafted a plan it submitted to the CDC in October detailing how it would distribute COVID-19 vaccine doses.
More: Johnson & Johnson CEO says the virus is the only competition in COVID-19 vaccine race
More: Health care workers to get first COVID-19 vaccine doses in Michigan
Although spokeswoman Lynn Sutfin said the plan is still being revised, it shows that Michigan health care workers would get vaccinated first. Any doses the state receives initially would be delivered to 143 hospitals and health systems for that purpose.
Who will be the first to get COVID-19 vaccines? (Photo: PETER HAMLIN/THE ASSOCIATED PRESS)
After that, doses would be distributed to 45 local health departments, which would then stand up their own clinics to provide vaccines to vulnerable populations, including residents of long-term care facilities, first responders, and those ages 65 and older who are at highest risk of severe illness with a COVID-19 infection.
How many doses will Michigan get?
That remains unclear, Sutfin said.
“The exact number that will be allocated to Michigan is not known,” she told the Free Press on Monday.
“We are actively preparing with hospitals and local health departments so that the state can distribute and administer vaccines once they become available. We have heard this could be as early as mid-December, but no date has been finalized at the federal level.”
What are the distribution challenges?
Both the Pfizer/BioNTech and Moderna vaccines use brand new technology that’s never before been approved by the FDA. They both use messenger RNA and must be frozen for storage.
Moderna’s vaccine can be safely stored for up to six months at minus 4 degrees Fahrenheit, which is regular freezer temperature. It remains stable at standard refrigerator temperatures of 36-46 degrees Fahrenheit for 30 days. And it can be kept at room temperature for up to 12 hours, making Moderna’s vaccine easier to store and distribute than Pfizer’s.
Pfizer’s COVID-19 vaccine candidate is more challenging in that it requires ultra-cold storage of minus 94 degrees Fahrenheit. It can be stored at 36-46 degrees Fahrenheit for up to 24 hours or at room temperature for no more than two hours after it thaws.

Henry Ford Health Systems has installed a dozen specialized freezers to store two promising COVID-19 vaccines, which could arrive in Detroit as early as Dec. 12, 2020. (Photo: Henry Ford Health Systems)
How will Pfizer ship its ultra-cold vaccines?
The company developed thermal kits containing dry ice that can be used to keep vials of the vaccine ultra cold for up to 15 days of safe storage while they’re shipped. Most will come from the company’s Kalamazoo manufacturing site, Pfizer said in a news release.
If the FDA grants emergency use authorization of its vaccine, the company says it will deliver the kits by air to major hubs throughout the country, and then distribute them by ground transport directly to sites where the vaccines will be administered.
“We will utilize GPS-enabled thermal sensors with a control tower that will track the location and temperature of each vaccine shipment across their preset routes. These GPS-enabled devices will allow Pfizer to proactively prevent unwanted deviations and act before they happen,” the company said.
Pfizer’s COVID-19 vaccine is being shipped in specially designed, insulated containers that hold between 195 and 975 five-dose vials and are about the size of a carry-on suitcase. The vials are stored in flat, pizza box-sized compartments, each of which holds 195 vials. A fully-loaded thermal container, which is reusable, contains five of these and weighs about 70 pounds. (Photo: Pfizer Inc.)
Will Michigan hospitals be able to store ultra-cold doses of Pfizer’s vaccines?
At least 31 hospitals and 11 local health departments now have ultra-cold freezer capabilities, Sutfin said, which is important for longer term storage of the Pfizer vaccine.
However, she said, “it is important to note that we expect the vaccine to be given to people very quickly after it is received, and they will be shipped in storage containers with dry ice that can be refreshed and will maintain the appropriate vaccine temperature. Thus, not every entity needs to have an ultra-cold freezer if they are able to receive and get the vaccine administered quickly, which is our expectation.”
Are there any other COVID-19 vaccines in the works?
Yes. There are dozens of other coronavirus vaccines in Phase 3 trials worldwide, and several of them are underway in the United States.
Among them are AstraZeneca’s investigational vaccine created with researchers at the University of Oxford and its spinoff company, Vaccitech.

Vials with COVID-19 vaccine stickers attached and syringes are pictured in front of University of Oxford and AstraZeneca logos. (Photo: JUSTIN TALLIS, AFP via Getty Images)
The late-stage study of the vaccine, AZD1222 is underway at Beaumont’s Royal Oak hospital and at Michigan Medicine at the University of Michigan.
In addition, Janssen Pharmaceutical Companies, a division of Johnson & Johnson, also is in Phase 3 trials with a potential COVID-19 vaccine. Henry Ford Health System, Michigan Medicine and Cherry Health are partnering with Janssen in the Phase 3 trial, which is called the Ensemble study.
Both AstraZeneca’s vaccine candidate and Janssen’s are recombinant vector vaccines created by modifying the adenovirus, which causes the common cold, with the genetic spike protein found in SARS-CoV-2. It’s the same method used to create an Ebola vaccine that has recently been granted authorization by the European Commission.
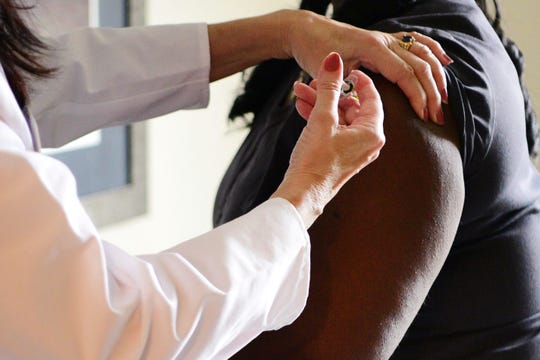
A woman receives an injection during phase 3 testing for the Janssen Pharmaceutical-Johnson & Johnson vaccine for COVID-19. (Photo: [Johnson & Johnson via AP])
Early data from AstraZeneca’s Phase 2/3 trials in the UK and Brazil showed its vaccine candidate ranges from 62% to 90% effective at preventing COVID-19, depending on how it is administered, and no hospitalizations or severe illnesses were reported among those who received the vaccine.
Contact Kristen Shamus: kshamus@freepress.com. Follow her on Twitter @kristenshamus.
Read or Share this story: https://www.freep.com/story/news/health/2020/12/01/covid-19-vaccine-michigan-pfizer-moderna/6462281002/
as per our monitoring this Story originally appeared * : ) here → *
How soon can Michiganders get a COVID-19 vaccine? Answering your biggest questions
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic
Add Story/ Your Say Until the Cause is Concluded
[*Select Category/Tag:
Coronavirus Covid-19 Pandemic
at Your Next Publish Screen.]

Johns Hopkins Coronavirus Resource Center
Johns Hopkins Coronavirus Resource Center
CBS News: What is a coronavirus testing desert?
CBS News correspondent Mark Strassmann spoke with Jennifer Nuzzo, a senior scholar at the Johns Hopkins Center for Health Security, about areas where people don’t have access to coronavirus tests, known as testing deserts.
as per our monitoring this Story originally appeared * : ) here → *
Johns Hopkins Coronavirus Resource Center
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic

Doctors behind viral COVID-19 misinformation video met with Vice President Pence
Doctors behind viral COVID-19 misinformation video met with Vice President Pence
The Week
Flynn suggests Trump deploy the military in ‘swing states’ to ‘rerun’ the election, soft-pedals martial law
Former National Security Adviser Michal Flynn tiptoed once again toward urging President Trump to declare martial law to overturn President-elect Joe Biden’s victory, citing discredited voter fraud claims. Trump “could immediately, on his order, seize every single one of these voting machines,” Flynn told Newsmax host Greg Kelly Thursday night. “He could order the, within the swing states, if he wanted to, he could take military capabilities, and he could place those in states and basically rerun an election in each of those states.”Using the U.S. military to force states to redo an election is “not unprecedented,” Flynn added. “These people are out there talking about martial law like it’s something that we’ve never done. Martial law has been instituted 64 times, Greg. So I’m not calling for that. We have a constitutional process,” and “that has to be followed.”> Here’s Michael Flynn on Newsmax saying that Trump could order “military capabilities” to swing states and “rerun an election in each of those states.”> > “People out there talk about martial law like it’s something that we’ve never done. Martial law has been instituted 64 times.” pic.twitter.com/KNmiAGGiPF> > — Aaron Rupar (@atrupar) December 18, 2020The federal government hasn’t implemented martial law since after Japan bombed Pearl Harbor in 1941, and then only in the territory of Hawaii. “Flynn’s insane rant” appears to rely on “the numerous invocations of martial law” before and during the Civil War, University of Texas law professor Steve Vladek said. Since then, Supreme Court precedents and several laws — notably the Posse Comitatus Act — have severely constrained the president’s ability to declare martial law.”Nothing to see here,” Slate’s Will Saletan tweeted. “Just a retired general and former undeclared foreign agent for an authoritarian regime, freshly pardoned by the president for lying to the FBI about his contacts with Russia, asserting precedents for martial law to overturn the president’s electoral defeat.”Former President Barack Obama fired Flynn as head of the Defense Intelligence Agency, then “tried to warn Trump about him,” New York Times reporter Maggie Haberbman noted. “Trump hired him, fired him, complained about him and his son during transition, and then has obviously changed course.” The Bulwark’s Tim Miller added: “Michael Flynn lying to the FBI was the biggest break the nation caught during the Trump years. The thought of the insane mad man in the room when Trump was making decisions is frightening.”More stories from theweek.com 5 insanely funny cartoons about Trump’s election-fraud failure Trump has reportedly been convinced he actually won, tells advisers he may not vacate the White House Pfizer says ‘millions’ of vaccine doses are waiting to be shipped — but the government hasn’t told them where to go
as per our monitoring this Story originally appeared * : ) here → *
Doctors behind viral COVID-19 misinformation video met with Vice President Pence
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic
Russia wants to distribute its questionable coronavirus vaccine to other countries: report
Russia wants to distribute its questionable coronavirus vaccine to other countries: report
 © Mikhail Svetlov/Getty Images Russian President Vladimir Putin Mikhail Svetlov/Getty Images
© Mikhail Svetlov/Getty Images Russian President Vladimir Putin Mikhail Svetlov/Getty Images
- Russian President Vladimir Putin wants to distribute its questionable Sputnik V coronavirus vaccine to other countries, Reuters reported Saturday.
- Russia first approved Sputnik V in August, but experts and health officials were skeptical the vaccine would work since it did not go through necessary phase 3 trials.
- The Kremlin also kept important information relevant to the vaccine’s success out of the public’s eye. This included its methodology.
- Visit Business Insider’s homepage for more stories.
Russian President Vladimir Putin said Saturday that the country is hoping to distribute its controversial Sputnik V coronavirus vaccine to other countries, according to Reuters.
Russia announced a successful coronavirus vaccine in August, but Sputnik V was approved under questionable circumstances. It was released before it went through phase 3 trials. In the United States, phase 3 is a requirement before a drug or vaccine can be vetted and approved by the Food and Drug Administration.
As Business Insider’s Susie Neilson previously reported, the vaccine’s early-trial results had not undergone peer review. Russia had also not revealed its methodology, further enshrouding Sputnik V in secrecy.
The rushed timeline led health officials to speculate whether the Kremlin coerced vaccine makers into putting out Sputnik V quickly to gain a leg up in the global race for a cure to the coronavirus.
Speaking at the annual G20 Summit, Putin said Saturday that Russia is in the process of creating a second and third vaccine in response to the coronavirus, Reuters reported Saturday.
Video: ‘Bursting with antibodies’: U.K. prime minister in quarantine again (NBC News)
What to watch next
UP NEXT
Putin also said restated his goal to mass-produce the vaccine for other countries. For months, the Russian president has been pushing other countries to take his vaccine seriously.
Russia in August said it would start the mass production process in September, despite the uncertainty that plagued Sputnik V.
Earlier this month, the Kremlin announced that preliminary data showed the vaccine had a 92% effectiveness rate at preventing the coronavirus. But the data was based on just 20 confirmed COVID-19 cases, according to a press release.
Putin told news reporters in August that he had offered to help the United States develop a coronavirus vaccine, but the White House declined.
Putin, however, maintains the assertion that the vaccine has “passed all the needed checks,” even adding at one point that he had his own daughter take it.
There are two coronavirus vaccines that have proven to have a high success rate at fighting the coronavirus. Pharmaceutical company Pfizer and biotech group Moderna announced earlier this month that they’ve developed vaccines with at least a 94.5% success rate at preventing the coronavirus in clinical trials.
Read the original article on Business Insider
Microsoft may earn an Affiliate Commission if you purchase something through recommended links in this article.
as per our monitoring this Story originally appeared * : ) here → *
Russia wants to distribute its questionable coronavirus vaccine to other countries: report
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic

Indiana coronavirus updates for Thursday, Dec. 10, 2020
Indiana coronavirus updates for Thursday, Dec. 10, 2020
INDIANAPOLIS — Thursday’s latest updates on the coronavirus pandemic:
IPS does not plan to return to in-person learning on January 4
Indianapolis Public Schools released the following statement regarding the Marion County Health Department’s announcement that in-person learning can resume on January 4.
“IPS does not anticipate shifting back to in-person learning on January 4 at this time. We believe it will be critical to assess the conditions and data after the Winter Break in order to make a determination if it is safe to return to in-person learning prior to the already scheduled January 19 date.
The safety of our students and staff continues to be our top priority. We will continue to leverage our return to in-person learning framework approved by the Board of School Commissioners in October.”
Marion County leaders giving update
Indianapolis Mayor Joe Hogsett and MCPHD Director Dr. Virginia Caine are giving an update on COVID-19 in Marion County.
Hogsett opened by saying the city needs help from the federal government to keep families financial afloat during the pandemic, noting that there has been no follow-up to the CARES Act passed in March. The funds distributed from the CARES Act will stop at the end of the year. He encouraged people to contact the leaders of Congress and demand action.
Dr. Caine recommended students and teachers stay away from people outside their household for 10 days before returning to in-person schooling next year. Caine said the executive order on closing schools was initially set to go through Jan. 18, but now schools may return to in-person learning on Jan. 4.
Caine said her office felt comfortable continuing to have bars open at 25 percent capacity after looking at the statistics and science, while restricting education to virtual only.
Ellen DeGeneres tests positive
Talk show host Ellen DeGeneres announced Thursday that she has tested positive for COVID-19.
“Fortunately, I’m feeling fine right now. Anyone who has been in close contact with me has been notified, and I am following all proper [Centers for Disease Control and Prevention] guidelines,” DeGeneres said in a statement posted on Twitter.
“I’ll see you all again after the holidays. Please stay healthy and safe,” she said in her statement.
ISDH daily update
The Indiana State Department of Health is reporting 6,604 more positive cases and 96 additional deaths from COVID-19. That brings Indiana’s totals to 404,935 cases and 6,302 confirmed deaths. Another 301 deaths are considered “probable,” meaning COVID-19 is believed to have contributed to a patient’s death, but there was no positive COVID test result on file.
The positivity rate remains high: 13.9 percent for all tests between Nov. 27 and Dec. 3, and 26.7 percent for unique individuals in the same time span.
More than 43 percent of the state’s ICU beds are in use by COVID patients. There are just over 20 percent of ICU beds available. On Wednesday, there were 3,221 people being treated for coronavirus in Indiana hospitals.
Moderna begins testing COVID-19 vaccine in US adolescents
Moderna announced Thursday that it has started testing its coronavirus vaccine candidate in adolescents.
The company, which made one of two vaccines expected to be approved soon for adult use in the U.S., said it plans to enroll 3,000 U.S. kids as young as 12 in the trial.
So far, there have only been a handful of attempts around the world to start exploring if any of the experimental coronavirus shots being pushed for adults also can protect children. Some U.S. pediatricians are worried they may not know if any of the shots work for young children in time for the next school year.
In a statement, Moderna Chief Executive Officer Stéphane Bancel said they hope to have data in the spring that can support giving the COVID-19 vaccine to children before the start of the 2021 school year.
Each child in the Moderna trial will either receive the COVID-19 candidate or a placebo at both vaccinations.
Indiana house speaker tests positive
Indiana House Speaker Todd Huston has tested positive for COVID-19. He got the result Wednesday and is experiencing mild symptoms.
“Unfortunately, I have tested positive for COVID-19,” Huston said in a statement. “I will continue quarantining at home and taking all necessary precautions. I look forward to returning to work when it’s safe to do so.”
Huston has not been in contact with any colleagues or staff members. He has not been to the Indiana Statehouse in the last week.
US jobless claims jump to 853,000
The number of people applying for unemployment aid jumped last week to 853,000, the most since September, evidence that companies are cutting more jobs as new virus cases spiral higher.
The Labor Department said Thursday that the number of applications increased from 716,000 the previous week. Before the coronavirus paralyzed the economy in March, weekly jobless claims typically numbered only about 225,000.
The latest figures coincide with a surging viral outbreak that appears to be weakening the job market and the economy and threatening to derail any recovery. Consumers thus far haven’t spent as much this holiday shopping season as they have in previous years, according to credit and debit card data. And in November, employers added jobs at the slowest pace since April. Restaurants, bars and retailers all cut jobs last month.
The total number of people who are receiving state-provided unemployment aid rose for the first time in three months to 5.8 million, the government said, from 5.5 million. That suggests that some companies have sharply pulled back on hiring.
All told, more than 19 million people are still dependent on some type of unemployment benefit. And unless Congress acts soon, nearly half of them will lose that aid in just over two weeks. That’s when two jobless aid programs that the federal government created in the spring are set to expire.
Indiana reinstating surgery limits amid COVID-19 surge
Indiana Governor Eric Holcomb is reimposing restrictions on hospitals from performing elective surgeries to free up hospital capacity with the state’s steep recent increases in serious COVID-19 illnesses.
Holcomb announced Wednesday that hospitals were being directed to postpone all non-urgent in-patient surgeries beginning Dec. 16 through Jan. 3.
Holcomb said Indiana is “on fire” with coronavirus spread.
The state halted elective medical procedures in April to help preserve hospital equipment and protective gear. Holcomb said he was extending the statewide mask order and toughening restrictions on crowd sizes that he reinstated last month.
Marion Co. COVID-19 update scheduled for 2 p.m.
Indianapolis Mayor Joe Hogsett and Marion County Public Health Department Director Dr. Virginia Caine will provide an update regarding Marion County’s current COVID-19 data on Thursday, Dec. 10 at 2 p.m.
WTHR will stream the news conference live here and on Facebook.
Pfizer COVID-19 vaccine faces last hurdle before US decision
Pfizer’s COVID-19 vaccine faces one final hurdle before an expected decision to greenlight the shot for use in millions of Americans. Food and Drug Administration advisers meet Thursday to scrutinize the company’s data for any red flags or oversights.
The public review comes as U.K. regulators investigate two apparent cases of allergic reaction to the vaccine. Safety will be top of mind for the panel of medical experts, who will vote on whether to endorse the vaccine.
They will also address unknowns about the vaccine’s effectiveness in certain groups. A final FDA decision and the first shots could follow within days.
Latest US, world numbers
There have been more than 15.3 million confirmed cases of COVID-19 in the U.S. as of 3:30 a.m. ET Thursday, according to Johns Hopkins University. There have been more than 289,400 deaths and 5.88 million people recovered.
Worldwide, there have been more than 68.9 million confirmed cases with more than 1.57 million deaths and 44.4 million recoveries.
The real number of people infected by the virus around the world is believed to be much higher — perhaps 10 times higher in the U.S., according to the Centers for Disease Control and Prevention — given testing limitations and the many mild cases that have gone unreported or unrecognized.
For most people, the coronavirus causes mild or moderate symptoms. For some, especially older adults and people with existing health problems, it can cause more severe illness like pneumonia, or death.
Carmel tests wastewater to predict COVID-19 spikes
The City of Carmel continues to test its wastewater to determine the prevalence of Covid-19 infections within the community. These studies can show an increase of the virus in the community up to 10 days ahead of a possible spike in positive cases. Wastewater samples are collected at the Carmel Wastewater Treatment Plant at 96th St. and the White River, then analyzed for the virus.
Mayor Jim Brainard said the city is taking this extra step to help local hospitals respond to a growing number of Covid-19 patients. Test data will allow hospitals to anticipate staffing needs and allocation of pharmaceuticals, along with scheduling elective procedures.
To test for COVID-19, copies of viral RNA (DNA) are pulled from the wastewater and quantified so that researchers may trend the numbers and determine if there are spikes or decreases in the wastewater concentrations.
as per our monitoring this Story originally appeared * : ) here → *
Indiana coronavirus updates for Thursday, Dec. 10, 2020
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic

How soon can Michiganders get a COVID-19 vaccine? Answering your biggest questions
How soon can Michiganders get a COVID-19 vaccine? Answering your biggest questions
CLOSE![]()
Dr. Anthony Fauci said distribution of vaccines for the coronavirus could begin by December or early January. USA TODAY
In the midst of another deadly coronavirus surge in a pandemic that has already taken more than 267,000 American lives, including 9,134 in Michigan, there’s hope on the horizon in promising news about potential COVID-19 vaccines.
Massachusetts-based biotech company Moderna applied Monday for emergency use authorization from the U.S. Food and Drug Administration for its coronavirus vaccine candidate on the heels of more encouraging data from late-stage clinical trials that suggest it is 94% effective and prevents severe illness in people with COVID-19.
Moderna is the second company to request federal approval for a potential coroanvirus vaccine. Pfizer — with its German-based partner BioNTech — asked regulators for emergency use authorization Nov. 20 for its coronavirus vaccine candidate.
Clinical trials suggest Pfizer’s vaccine is 95% effective against COVID-19 beginning 28 days after the first dose.
How soon can I get a COVID-19 vaccine?
An FDA advisory committee is expected to meet Dec. 17 to review the potential Moderna vaccine’s safety and efficacy, the company announced Monday. If it’s approved, the first doses of the vaccine could be given as early as Dec. 21, the New York Times reported.
The same committee is scheduled to discuss the Pfizer COVID-19 vaccine candidate Dec. 10. Pfizer said in a news release that its vaccine could be ready for distribution “within hours” of getting approval.
“We could be seeing both of these vaccines out and getting into people’s arms before Christmas,” Alex Azar, secretary of the U.S. Department of Health and Human Services, said Monday on “CBS This Morning.”
But that doesn’t mean most people will be able to call their doctors to get a vaccination before the New Year.
There will be limited supply — especially initially — of any vaccine that gets emergency use authorization. It could be months before there’s enough available for the general public to get immunized.

Moderna’s experimental COVID-19 vaccine on Nov. 16, 2020, in Paris. (Photo: Joel Saget/AFP via Getty Images)
How effective are these COVID-19 vaccines?
Though neither company has published outcomes of its Phase 3 trials, early data from that research suggests the COVID-19 vaccine candidates are very effective.
In its Phase 3 trial of 30,000 people, Moderna’s vaccine candidate was shown to be 94.1% effective and prevents severe illness in people with COVID-19.
Of the 196 coronavirus cases evaluated among the people who took part in the trial, 185 were in the placebo group. Additionally, of the 30 people enrolled in the Moderna vaccine trial who had severe cases of COVID-19, all 30 were in the placebo group.
Clinical trials of Pfizer’s vaccine candidate suggest it is 95% effective against COVID-19 beginning 28 days after the first dose. Of 170 confirmed cases of COVID-19 evaluated in its Phase 3 trials of the Pfizer vaccine, the company reported 162 were among people in the group that got placebo. Eight cases were identified in the group that received the vaccine.
Are they safe?
Though safety reviews continue, so far no serious concerns have been identified for either Pfizer’s or Moderna’s COVID-19 vaccine candidates.
The most common side effects were injection site pain/redness, fever, fatigue, muscle and joint aches, and headache. Patients in the Moderna trials said the reactions were more pronounced after the second dose of the vaccine.

A vial of Pfizer’s mRNA COVID-19 Vaccine is prepared for first dosing at University of Maryland School of Medicine. (Photo: University of Maryland School of Medicine)
There was one COVID-19-related death in the Moderna vaccine study, but the person who died was in the group who received placebo rather than vaccine.
How many doses of the vaccine will each company produce?
Pfizer says it will produce up to 50 million doses of its vaccine globally in 2020 and up to 1.3 billion doses by the end of 2021.
Moderna also is scaling up its global manufacturing with an aim of delivering 500 million to 1 billion doses per year, beginning in 2021.
While those seem like huge numbers, it’s not enough to vaccinate the world’s population of roughly 7.8 billion people, which means doses will be distributed using priorities established by federal and state governments.
Additionally, both vaccines require two doses per person.
Who will get vaccinated first?
The U.S. Centers for Disease Control and Prevention is working with state public health officials to plan for distribution of COVID-19 vaccines.
The CDC’s Advisory Committee on Immunization Practices is planning an emergency session Tuesday to vote on a recommendation that health care providers should be vaccinated first, followed by residents of long-term care facilities, according to a STAT News report.
The Michigan Department of Health and Human Services drafted a plan it submitted to the CDC in October detailing how it would distribute COVID-19 vaccine doses.
More: Johnson & Johnson CEO says the virus is the only competition in COVID-19 vaccine race
More: Health care workers to get first COVID-19 vaccine doses in Michigan
Although spokeswoman Lynn Sutfin said the plan is still being revised, it shows that Michigan health care workers would get vaccinated first. Any doses the state receives initially would be delivered to 143 hospitals and health systems for that purpose.

Who will be the first to get COVID-19 vaccines? (Photo: PETER HAMLIN/THE ASSOCIATED PRESS)
After that, doses would be distributed to 45 local health departments, which would then stand up their own clinics to provide vaccines to vulnerable populations, including residents of long-term care facilities, first responders, and those ages 65 and older who are at highest risk of severe illness with a COVID-19 infection.
How many doses will Michigan get?
That remains unclear, Sutfin said.
“The exact number that will be allocated to Michigan is not known,” she told the Free Press on Monday.
“We are actively preparing with hospitals and local health departments so that the state can distribute and administer vaccines once they become available. We have heard this could be as early as mid-December, but no date has been finalized at the federal level.”
What are the distribution challenges?
Both the Pfizer/BioNTech and Moderna vaccines use brand new technology that’s never before been approved by the FDA. They both use messenger RNA and must be frozen for storage.
Moderna’s vaccine can be safely stored for up to six months at minus 4 degrees Fahrenheit, which is regular freezer temperature. It remains stable at standard refrigerator temperatures of 36-46 degrees Fahrenheit for 30 days. And it can be kept at room temperature for up to 12 hours, making Moderna’s vaccine easier to store and distribute than Pfizer’s.
Pfizer’s COVID-19 vaccine candidate is more challenging in that it requires ultra-cold storage of minus 94 degrees Fahrenheit. It can be stored at 36-46 degrees Fahrenheit for up to 24 hours or at room temperature for no more than two hours after it thaws.

Henry Ford Health Systems has installed a dozen specialized freezers to store two promising COVID-19 vaccines, which could arrive in Detroit as early as Dec. 12, 2020. (Photo: Henry Ford Health Systems)
How will Pfizer ship its ultra-cold vaccines?
The company developed thermal kits containing dry ice that can be used to keep vials of the vaccine ultra cold for up to 15 days of safe storage while they’re shipped. Most will come from the company’s Kalamazoo manufacturing site, Pfizer said in a news release.
If the FDA grants emergency use authorization of its vaccine, the company says it will deliver the kits by air to major hubs throughout the country, and then distribute them by ground transport directly to sites where the vaccines will be administered.
“We will utilize GPS-enabled thermal sensors with a control tower that will track the location and temperature of each vaccine shipment across their preset routes. These GPS-enabled devices will allow Pfizer to proactively prevent unwanted deviations and act before they happen,” the company said.
Pfizer’s COVID-19 vaccine is being shipped in specially designed, insulated containers that hold between 195 and 975 five-dose vials and are about the size of a carry-on suitcase. The vials are stored in flat, pizza box-sized compartments, each of which holds 195 vials. A fully-loaded thermal container, which is reusable, contains five of these and weighs about 70 pounds. (Photo: Pfizer Inc.)
Will Michigan hospitals be able to store ultra-cold doses of Pfizer’s vaccines?
At least 31 hospitals and 11 local health departments now have ultra-cold freezer capabilities, Sutfin said, which is important for longer term storage of the Pfizer vaccine.
However, she said, “it is important to note that we expect the vaccine to be given to people very quickly after it is received, and they will be shipped in storage containers with dry ice that can be refreshed and will maintain the appropriate vaccine temperature. Thus, not every entity needs to have an ultra-cold freezer if they are able to receive and get the vaccine administered quickly, which is our expectation.”
Are there any other COVID-19 vaccines in the works?
Yes. There are dozens of other coronavirus vaccines in Phase 3 trials worldwide, and several of them are underway in the United States.
Among them are AstraZeneca’s investigational vaccine created with researchers at the University of Oxford and its spinoff company, Vaccitech.

Vials with COVID-19 vaccine stickers attached and syringes are pictured in front of University of Oxford and AstraZeneca logos. (Photo: JUSTIN TALLIS, AFP via Getty Images)
The late-stage study of the vaccine, AZD1222 is underway at Beaumont’s Royal Oak hospital and at Michigan Medicine at the University of Michigan.
In addition, Janssen Pharmaceutical Companies, a division of Johnson & Johnson, also is in Phase 3 trials with a potential COVID-19 vaccine. Henry Ford Health System, Michigan Medicine and Cherry Health are partnering with Janssen in the Phase 3 trial, which is called the Ensemble study.
Both AstraZeneca’s vaccine candidate and Janssen’s are recombinant vector vaccines created by modifying the adenovirus, which causes the common cold, with the genetic spike protein found in SARS-CoV-2. It’s the same method used to create an Ebola vaccine that has recently been granted authorization by the European Commission.

A woman receives an injection during phase 3 testing for the Janssen Pharmaceutical-Johnson & Johnson vaccine for COVID-19. (Photo: [Johnson & Johnson via AP])
Early data from AstraZeneca’s Phase 2/3 trials in the UK and Brazil showed its vaccine candidate ranges from 62% to 90% effective at preventing COVID-19, depending on how it is administered, and no hospitalizations or severe illnesses were reported among those who received the vaccine.
Contact Kristen Shamus: kshamus@freepress.com. Follow her on Twitter @kristenshamus.
Read or Share this story: https://www.freep.com/story/news/health/2020/12/01/covid-19-vaccine-michigan-pfizer-moderna/6462281002/
as per our monitoring this Story originally appeared * : ) here → *
How soon can Michiganders get a COVID-19 vaccine? Answering your biggest questions
SCUC-CCP :: Selective Cause Until it is Concluded : Coronavirus Covid-19 Pandemic
*Above All Stories ON/ABOUT/FROM ‘Coronavirus Covid-19 Pandemic’ are Published/Edited/Enhanced randomly by the Global Open Profile: allaboutian.

 scroll for the Story
scroll for the Story
~ MORE ~
explore a.
→ all Story at a.
✐ Publish
?️?️ Perceive
? Play
TrendingStory
SocialStory
RandomStory

More SCUC
Suggested SCUC
Suggest SCUC
*If You think there is a certain Cause in this planet which should be concluded immediately. You may Suggest that here as ‘Suggested SCUC’.
*Select Category/Tag: Suggested SCUC , Put A Clear Title, Upload an Image & Give A brief/details Description to portray the Cause at your next publish screen.